Effects of Trichoderma and Bacillus pre-treatments on the germination of Agave victoriae-reginae SEEDS T. Moore
DOI:
https://doi.org/10.29298/rmcf.v13i69.844Keywords:
Agave, endangered species, dormancy, noha, germinative treatment, seed viabilityAbstract
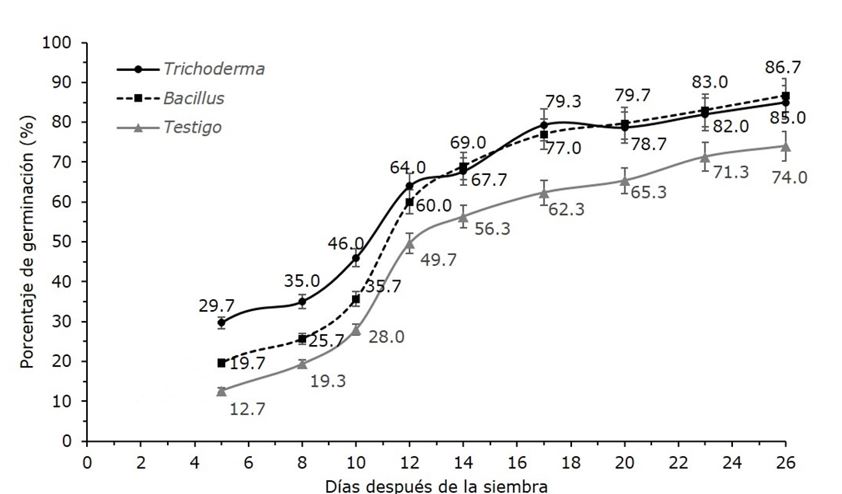
Application of germinative treatments is key to improve germination rates of forest species seeds, among which is found the use of microorganisms. In this study, the percentage of germination of Agave victoriae-reginae seeds treated with Trichoderma spp, and Bacillus spp was evaluated. Three treatments were tested: T1 (Trichoderma), T2 (Bacillus), and T3 (control), with three replications of 100 seeds each one. The seeds were immersed in a solution 1 × 106 CFU (treatments T1 and T2), and water in T3, then they were sowed, and the germination percentage was recorded daily. The germination began 5 days before the sown, which indicated that seeds did not present dormancy. Between 8° and 12° day an accelerated increase of germination was recorded in the three cases, until it ended at day 26, with 85 % as total germination for seeds treated with Trichoderma spp., 86.7 % with Bacillus spp, and 74 % with control. A significant effect of treatment on the germination percentage was found; even the use of both microorganisms accelerated the germination process compared to control. These results suggest that the use of Trichoderma spp, and Bacillus spp. as pregerminative treatments can improve the germination of A. victoriae-reginae and its long-term conservation, which contributes to the preservation of this endangered species.
Downloads
References
Ahmad, F., I. Ahmad and M. S. Khan. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological Research 163(2):173-181. Doi:10.1016/j.micres.2006.04.001.
Barnett, J. P. and S. Varela. 2004. A review of chemical treatments to improve germination of longleaf pine seeds. Native Plants Journal 5(1):18-24. Doi:10.2979/NPJ.2004.5.1.18.
Cabra C., T., C. A. Rodríguez G., C. P. Villota C., O. A. Tapasco A. y A. Hernández R. 2017. Efecto de Bacillus sobre la germinación y crecimiento de plántulas de tomate (Solanum lycopersicum L). Acta Biológica Colombiana 22(1):37-44. Doi:10.15446/abc.v22n1.57375.
Castillo R., F., J. D. Sánchez C., S. E. Rangel E. y J. Canul K. 2014. Efecto de microorganismos en la promoción de la germinación de semillas de la cactácea Echinocactus platyacanthus Link & Otto. Interciencia 39(12):863-867. www.redalyc.org/articulo.oa?id=33932786006 (10 de abril de 2020).
Castillo Q., D., D. Y. Avila F., F. Castillo R., A. Antonio B. y O. U. Martínez B. 2015. Nolina cespitifera Trel. recurso forestal no maderable de importancia económica y social del noreste de México. Interciencia 40(9):611-617. https://www.redalyc.org/articulo.oa?id=33940998005 (3 de mayo de 2020).
Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). 2017. Lista de especies CITES. https://bit.ly/3Fm2Esz (10 de agosto de 2020).
Cubillos H., J. G., A. Páez R. y L. Mejía D. 2011. Evaluación de la capacidad biocontroladora de Trichoderma harzianum Rifai contra Fusarium solani (Mart.) Sacc. asociado al complejo “Secadera” en Maracuyá, bajo condiciones de invernadero. Revista Facultad Nacional Agronomía Medellín 64(1): 5821-5830. http://www.scielo.org.co/pdf/rfnam/v64n1/a08v64n01.pdf (6 de mayo de 2020).
Delgado-Sánchez, P., M. A. Ortega-Amaro, J. F. Jiménez-Bremont and J. Flores. 2010. Are fungi important for breaking seed dormancy in desert species? Experimental evidence in Opuntia streptacantha (Cactaceae). Plant Biology 13(1):154-159. Doi:10.1111/j.1438-8677.2010.00333.x.
Delgado-Sánchez, P., J. F. Jiménez-Bremont, M. L. Guerrero-González and J. Flores. 2013. Effect of fungi and light on seed germination of three Opuntia species from semiarid lands of central Mexico. Journal of Plant Research 126: 643–649. Doi:10.1007/s10265-013-0558-2.
Díaz P., G., J. A. Ruíz C., G. Medina G., M. A. Cano G., V. Serrano A. e I. Sánchez C. 2007. Estadísticas climatológicas básicas del estado de Coahuila. (Periodo 1961 – 2003). Libro Técnico N° 16. Campo Experimental Cotaxtla. CIRGOC-INIFAP Medellín de Bravo, Ver., México. 159 p.
Donohue, K., L. Dorn, C. Griffith, E. Kim, A. Aguilera, C. R. Polisetty and J. Schmitt. 2005. The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution 59(4): 758-770. Doi: 10.1111/j.0014-3820.2005.tb01751.x.
Doria, J. 2010. Generalidades sobre las semillas: Su producción, conservación y almacenamiento. Cultivos Tropicales 31(1):74-85. http://scielo.sld.cu/pdf/ctr/v31n1/ctr11110.pdf (5 de junio de 2020).
Durán, M. C. y H. G. Núñez P. 2015. Utilización de un sistema de inmersión temporal (SIT) para multiplicar plantas ornamentales de Agave victoriae-reginae. Jóvenes en la Ciencia 1(2): 66-71. http://www.jovenesenlaciencia.ugto.mx/index.php/jovenesenlaciencia/article/view/381 (13 de junio de 2020).
Freeman, C. E. 1975. Germination responses of a New Mexico population of Parry agave (Agave parryi Engelm. var. parryi) to constant temperature, water stress and pH. The Southwestem Naturalist 20(1):69-74. Doi: 10.2307/3670012.
Freeman, C. E., R. S. Tiffany and W. H. Reid. 1977. Germination responses of Agave lechuguilla, A. parryi, and Fouquieria splendens. The Southwestern Naturalist 22(2):195-204. Doi: 10.2307/3669810.
García, E. 1973. Modificaciones al sistema de clasificación climática de Köppen, Segunda Edición. Instituto de Geografía, UNAM. México, D.F., México. 146 p.
Gentry, H. S. 1982. Agaves of Continental Norht American. The University of Arizona Press. Tucson, AZ, USA. 670 p.
Gómez R., M., J. Villegas, J., C. Sáenz R, C. y R. Lindig C. 2013. Efecto de la micorrización en el establecimiento de Pinus pseudostrobus en cárcavas. Madera y Bosques 19(3):51-63. Doi: 10.21829/myb.2013.193327.
González E., M. S., M. González E., I. L. López E., L. Reséndiz R., J. A. Tena F. y F. I. Retana R. 2011. El complejo Agave victoriae-reginae (Agavaceae). Acta Botánica Mexicana 95:65-94. Doi: 10.21829/abm95.2011.268.
Guillen, C. R., F. D. Hernández C., G. Gallegos M., R: Rodríguez H., C. N. Aguilar G., E. Padrón C. y M. H. Reyes V. 2006. Bacillus spp. as biocontrol in infested soils with Fusarium spp., Rhizoctonia solani Kühn and Phytophthora capsici Leonina and its effect on development and yield of pepper (Capsicum annuum L.). Revista Mexicana de Fitopatología 23:105-113. https://www.redalyc.org/articulo.oa?id=61224204 (9 de mayo de 2020).
Hernández M., S., R. Novo S., M. A. Mesa P., A. Ibarra M. y D. Hernández R. 2017. Capacidad de Trichoderma spp. como estimulante de la germinación en maíz (Zea mays L.) y frijol (Phaseolus vulgaris L.). Revista de Gestión del Conocimiento y el Desarrollo Local 4(1):19-23. https://revistas.unah.edu.cu/index.php/RGCDL/article/view/898 (10 de agosto de 2020).
Khurana, E. and J. S. Singh. 2001. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environmental Conservation 28(1):39-52. Doi:10.1017/S0376892901000042.
Moncaleano E., J., B. C. F. Silva, S. R. D. Silva, J. A. A. Granja, M. C. J. L. Alves and M. F. Pompelli. 2013. Germination responses of Jatropha curcas L. seeds to storage and aging. Industrial Crops and Products 44: 684-690. Doi:10.1016/j.indcrop.2012.08.035.
Moreno R., A., V. García M., J. L. Reyes C., J. Vásquez A. y P. Cano R. 2018. Rizobacterias promotoras del crecimiento vegetal: una alternativa de biofertilización para la agricultura sustentable. Revista Colombiana de Biotecnología 20(1):68-83. Doi: 10.15446/rev.colomb.biote.v20n1.73707.
Ortega, U., M. Duñabeitia, S. Menendez, C. González M. and J. Makada. 2004. Effectiveness of mycorrhizal inoculation in the nursery on growth and water relations of Pinus radiata in different water regimes. Tree Physiology 24: 65-73. Doi:10.1093/treephys/24.1.65.
Peña V., C. B., A. B. Sánchez U., J. R. Aguirre R., C. Trejo, E. Cárdenas and A. Villegas M. 2006. Temperature and mechanical scarification on seed germination of ´maguey´(Agave salmiana Otto ex Salm-Dyck). Seed Science and Technology 34: 47-56. Doi: 10.15258/sst.2006.34.1.06.
R Core Team. 2017. R project 4.3.4. https://www.r-project.org/ (16 de febrero de 2017).
Ramírez T., H. M., C. B. Peña V., J. R. Aguirre R., J. A. Reyes A., A. B. Sánchez U. and S. Valle G. 2012. Seed germination temperatures of eight Mexican Agave species with economic importance. Plant Species Biology 27:124-137. Doi: 10.1111/j.1442-1984.2011.00341.x.
Ramírez T., H. M., R. Niño V., J. R. Aguirre R., J. Flores, J. A. De-Nova V. and R. Jarquin G. 2016. Seed viability and effect of temperature on germination of Agave angustifolia subsp. tequilana and A. mapisaga; two useful Agave species. Genetic Resources and Crop Evolution 63: 881-888. Doi: 10.1007/s10722-015-0291-x.
Rangel L., S., A. Casas and P. Dávila. 2015. Facilitation of Agave potatorum: An ecological approach for assisted population recovery. Forest Ecology and Management 347:57-74. Doi: 10.1016/j.foreco.2015.03.003.
Sánchez U., A. B., I. Ortega, I. Cano, A. González, C. B. Peña V., G. Rivero, G. Sthormes y D. Pacheco. 2011. Efecto de la escarificación de la semilla y del sustrato sobre el crecimiento de plántulas de Agave salmiana. Revista de la Facultad de Agronomía 28: 40-50. https://produccioncientificaluz.org/index.php/agronomia/article/view/26979/27604 (20 de agosto de 2020).
Sánchez S., J., J. Flores, E. Jurado, J. Sáenz M., P. Orozco F. y G. Muro P. 2017. Hidrocoria en semillas de Agave victoriae-reginae T. Moore, especie en peligro de extinción: Morfología y anatomía como facilitadores de la hidro-dispersión y germinación. Gayana Botánica 74(2):251-261. Doi:10.4067/S0717-66432017000200251.
Secretaria de Medio Ambiente y Recursos Naturales (Semarnat). 2010. Norma Oficial Mexicana NOM-059-ECOL-2010. Protección ambiental Especies nativas de México de flora y fauna silvestres. Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio. Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010. México, D.F., México. 40 p.
http://dof.gob.mx/nota_detalle.php?codigo=5173091&fecha=30/12/2010 (5 de agosto de 2020).
Sieber, T. N. 2007. Endophytic fungi in forest trees: Are they mutualist? Fungal Biology Review 21: 75-89. Doi: 10.1016/j.fbr.2007.05.004.
Tropicos. 2019. Tropicos.org. Missouri Botanical Garden. http://www.tropicos.org. (6 de agosto de 2020).
Published
Versions
- 2022-03-31 (2)
- 2022-01-10 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Forestales

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Forestales accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Forestales recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access.
All the texts published by Revista Mexicana de Ciencias Forestales –with no exception– are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Forestales (for example, include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Forestales.
For all the above, the authors shall send the form of Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: ciencia.forestal2@inifap.gob.mx
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.





